Wayne State’s Jitao Zhang publishes new research to bring Brillouin microscopy closer to widespread use in diagnostic medicine
Diagnostic imaging offers physicians and scientists critical visual representations of internal body structures, greatly enhancing clinical analysis and medical intervention. Researchers continue to break new ground on how various imaging technologies can provide a better understanding of human health.
Jitao Zhang, assistant professor of biomedical engineering (BME) at Wayne State University and a scientific member of the Karmanos Cancer Institute’s Molecular Imaging Program, is an award-winning researcher who holds three patents on a novel imaging technique called Brillouin microscopy that can map cell and tissue stiffness often associated with early signs of such diseases as cancer and Alzheimer's.
Different from conventional imaging methods such as confocal fluorescence microscopy, Brillouin microscopy can acquire the mechanical information (e.g., stiffness and viscosity) of biological samples in a non-contact and label-free manner.
His lab's work to improve this method, which can answer many important questions in biophysics and mechanobiology, was featured in The Guardian after being named by peers in the scientific community as one of the 10 biggest science stories of 2022.
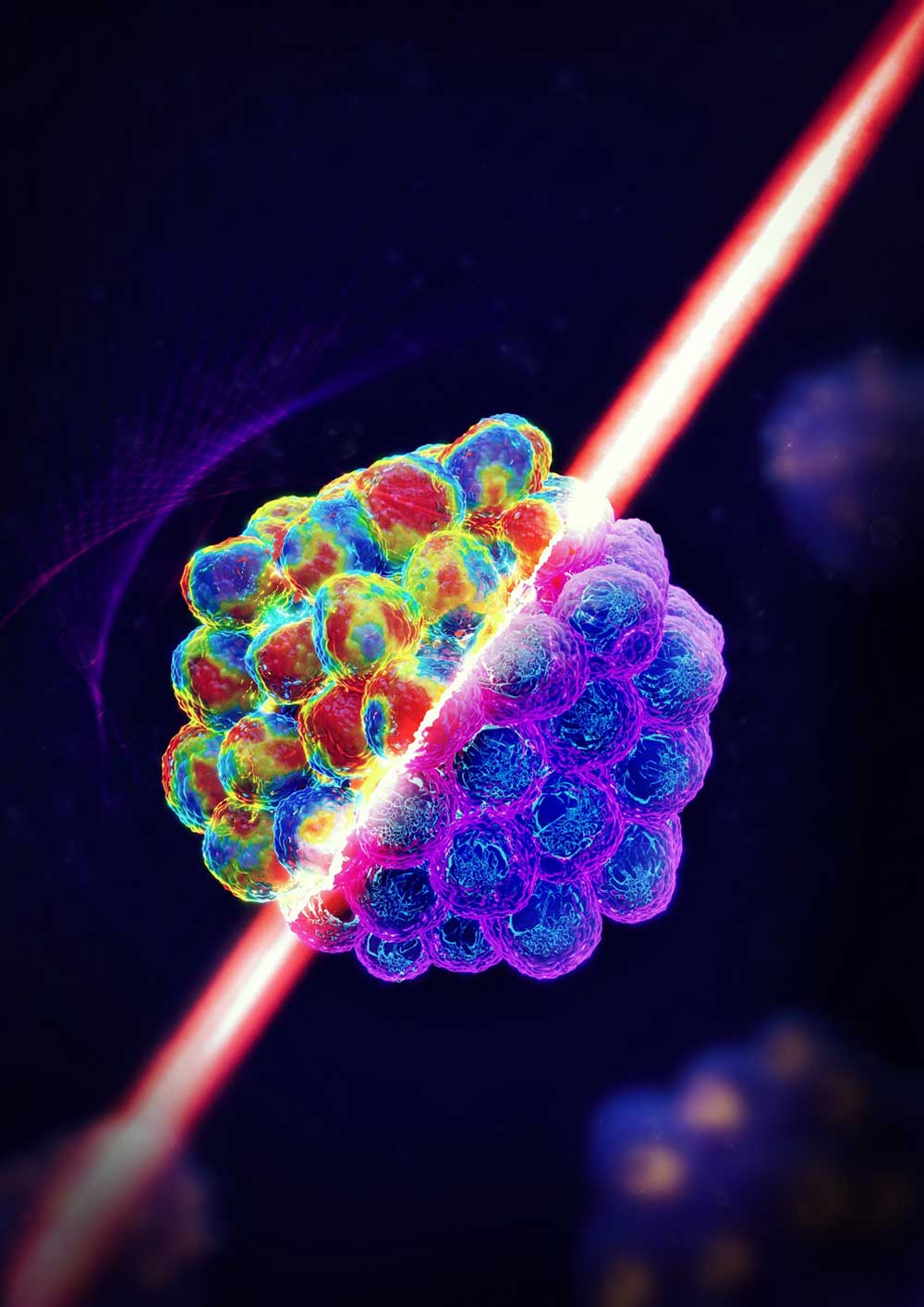
Zhang and his collaborators from the University of Maryland — where Zhang spent six years in the Department of Bioengineering before joining Wayne State in 2021 — and the National Institutes of Health (NIH) recently published a research article in the March issue of Nature Methods examining the use of dual line-scanning Brillouin microscopy (dLSBM) to improve acquisition speed and reduce irradiation doses, two main limiting factors to the widespread use of this technique in biomedicine.
“Existing confocal Brillouin microscopy is fairly slow; it takes a few minutes to acquire one mechanical image of a single cell,” said Zhang, whose research is funded and supported by such agencies as the NIH, the National Science Foundation and the American Cancer Society. “If we are imaging larger samples such as tumor cell clusters or early-stage embryo, we need to wait an hour or longer to obtain one image.”
Using dLSBM, Zhang’s team reported speeds of 50 to 100 times faster than its counterpart, while reducing the light irradiation level by 80 times for 2D and 3D mechanical mapping.
“With this innovation, we can acquire one mechanical image of cell clusters in a few minutes,” he said. “This improved acquisition speed is important because it allows us to investigate details of cell behaviors in almost real time.”
Brillouin microscopy is an optical imaging modality rooted in what is known as Brillouin light scattering (BLS), first reported in 1922 by French physicist Léon Brillouin. BLS occurs when light interacts with a substance and thermal fluctuations or vibrations of molecules in the material cause the light to scatter. Vibrations can be affected by certain factors, including heat, compression, water content or material stiffness. It is the latter of these characteristics that is most valuable for the application of Brillouin microscopy as a diagnostic tool.
Disease progression, such as cancer metastasis, is often associated with changes in cell stiffness, but this is difficult to measure because cells are small and live in very soft tissue. Conventional techniques measure prepared cells on a petri dish or other hard substrate. A Brillouin microscope only uses a laser beam to probe the mechanical properties, allowing measurement to be conducted when cells are in their physiological conditions.
Since physical contact is not needed, Brillouin technology is far less invasive and more convenient. Another application for which these characteristics are important is to better understand embryonic tissue development, particularly as it relates to birth diseases and disorders.
“Due to the 3D structure of an embryo, traditional contact-based techniques encounter big challenges for in vivo measurement,” said Zhang. “Since Brillouin microscopy works in a non-contact manner, it sometimes becomes the only available choice.”
Zhang collaborates with biologists and doctors at Karmanos and other institutions to address biomedical questions with technological innovations. However, Zhang noted that “Brillouin technology is still in its early stage and has limited imaging depth. Our lab will continue to work on making it more accessible for broader biomedical fields.”
The interaction between engineers and members of the medical community is especially critical at the diagnostic stage of the health care journey. Zhang and other Wayne State BME researchers are steering biomedical research and health care to levels of unparalleled progression.
This work was supported by grants from the National Science Foundation (DBI-1942003, CMMI-1929412) to collaborator Giuliano Scarcelli (G.S.) from University of Maryland, the National Institutes of Health (R21CA258008 to G.S., R01EY028666 to G.S., R01HD095520 to G.S., K25HD097288 to Zhang (J.Z.)), the Intramural Research Program of the National Cancer Institute to collaborator Kandice Tanner, NIH, the American Cancer Society Institutional Research Grant (1816016) to J.Z. and a Wayne State University Research Grant to J.Z.